Jul 30, 2025
Opportunities in Health Business: Integrating Herbal Remedies into Medical Devices
Explore the rising trend of combining herbal remedies with medical devices. Discover top products, benefits, and global opportunities in the health market.
รายละเอียดข่าวสารทั้งหมด

Top Supplement Extracts for 2025–2026: A Golden Opportunity for Health-Forward Entrepreneurs
As global awareness around health and wellness continues to rise, the dietary supplement industry is booming. By 2034, the global supplement market is projected to reach USD 402.20 billion, with a CAGR of 7.87%. Meanwhile, the market for food supplement ingredients is expected to grow from USD 17.26 billion in 2025 to USD 29.70 billion by 2032, reflecting a CAGR of 8.06%.
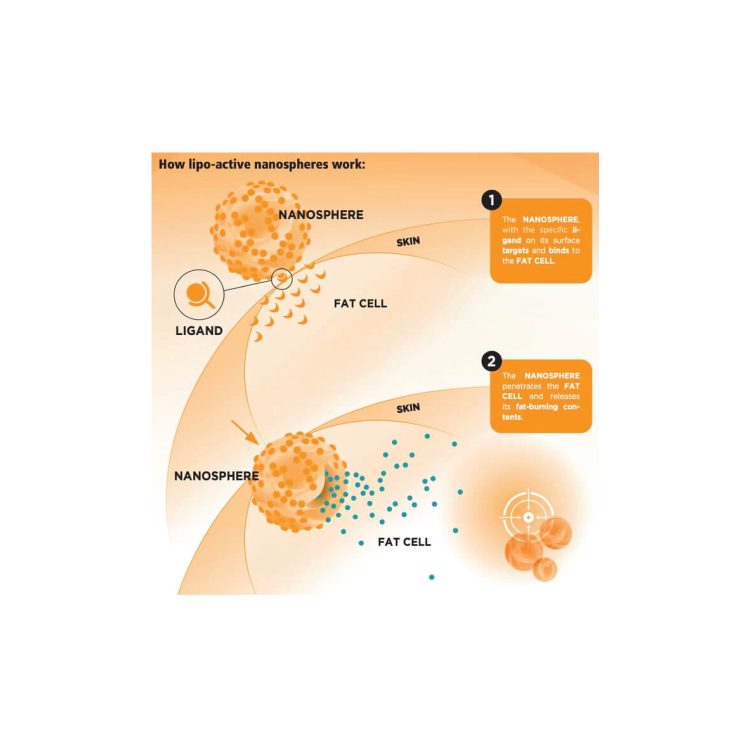
Supplement Innovation 2025: New Technologies to Boost Nutrient Absorption
In 2025, the dietary supplement industry is entering a new era—driven by cutting-edge technologies that significantly enhance nutrient absorption in the human body. These advancements aim to ensure consumers get the maximum benefits from high-quality, natural, and safe ingredients. Below are some of the most promising innovations shaping the future of supplements:

Advantages of Manufacturing Medical Devices with an ISO 13485-Certified Factory
Manufacturing medical devices requires precision and strict quality standards to ensure the products are safe and effective for end users. With the global healthcare and wellness markets rapidly growing