Manufacturing products in the medical device category requires obtaining authorization from the Thai FDA (Food and Drug Administration), specifically from the Medical Device Division. Products are classified into various categories such as:
• General Medical Devices (Class I) – e.g., herbal compresses that make no therapeutic claims
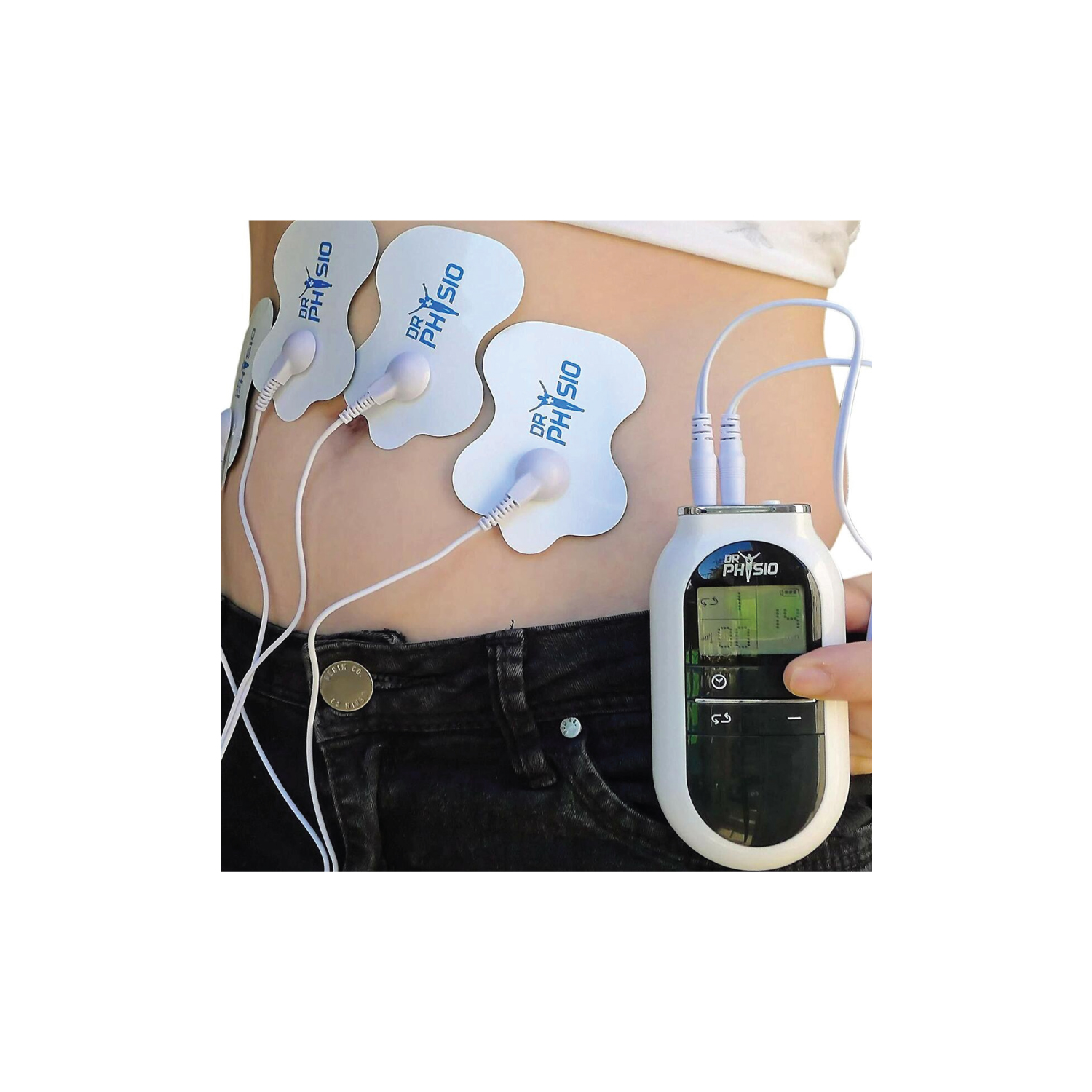
• Controlled Medical Devices (Class II / III) – e.g., electric massagers
If you’re producing a massage oil that contains no active ingredients and is used solely to reduce friction during massage, it can also be registered as a medical device—as long as no claims are made regarding disease treatment.
Why Start Now?
• Clear Market Trend: Consumers are demanding high-quality health and wellness products
• Low Initial Investment: OEM manufacturing of medical devices doesn’t require a huge upfront cost, but offers great potential to build a strong brand
• Government Support: Thai policy encourages health innovations, with an open opportunity in export markets
2025: A Golden Opportunity
This year presents a golden opportunity for entrepreneurs to enter the medical device market—especially in the Wellness and Health Recovery sectors, which are rapidly gaining popularity.
OEM manufacturing is an ideal entry strategy because it allows:
• Custom formulation
• Unique product design
• Full quality control
Start today to grow your brand alongside the global wellness trend.